SHRI SHIVAJI SCIENCE COLLEGE, AMRAVATI
DBT STAR COLLEGE PROJECT ACTIVITY
ACTIVITY REPORT

Estimation of concentration of electrolytes in mixture
Activity Dates: 24th March 2025 to 28th March 2025
Type of Activity: Students Workshop
Organizing Department: Department of Chemistry
Program Coordinators: Dr. V.B. Khobragade Ms. K.V. Kumbhalkar
Head of the Department: Dr. P.R. mandlik
External Collaborator (if any): Nil
Objectives:
- To learn that electrolytes are substances that conduct electricity when dissolved in a solvent, due to the presence of charged ions.
- To know different Analytical Techniques for Concentration Determination of mixtures.
- To understand how conductivity measurements can be used to determine the concentration of electrolyte solutions based on their ability to conduct electricity.
- To measure the potential or current generated by the interaction of the electrolyte with an electrode.
- To explore the use colorimetry or spectrophotometry to measure the absorbance of coloured electrolyte solutions and relate it to their concentration.
No of Beneficieries: 100
Classes Involved: B.Sc. II students
Venue of the Activity: Chemistry Laboratory
Activity Report:
“Estimation of concentration of electrolytes in mixture.”
Organized by: Shri Shivaji Science College, Amravati
Assisted by: Department of Chemistry
Date: 24th March – 28th March 2025
Eligibility: B.Sc. II ( SEM IV) Students (PCM, PHG, MEB, BIO Groups)
Number of Beneficiaries: 100
Introduction: The Department of Chemistry at Shri Shivaji Science College, Amravati, successfully organized a five-day DBT Star College Activity aimed at Estimation of concentration of electrolytes in mixtures. By performing experiments to estimate the concentration of electrolytes, students gain a practical understanding of how these solutions behave and how their concentration can be determined. They learn about various analytical techniques and the importance of electrolytes in different applications, from chemistry to biology and medicine.
Objectives:
• Nature of Electrolytes:
Students learn that electrolytes are substances that conduct electricity when dissolved in a solvent, due to the presence of charged ions.
• Types of Electrolytes:
They become familiar with different types of electrolytes, including strong and weak electrolytes, and how their behavior differs in solution.
• Electrolyte Concentration:
Students understand that concentration refers to the amount of solute (electrolyte) present in a given amount of solvent or solution.
• Students come to know the different Analytical Techniques for Concentration Determination of mixtures.
• Titration:
Students learn how to use titration to accurately determine the concentration of an electrolyte solution by reacting it with a known standard solution.
• Conductometry or Conductivity Measurements:
Students understand how conductivity measurements can be used to determine the concentration of electrolyte solutions based on their ability to conduct electricity.
• Potentiometry or Potential measurement :
Students understand how to measure the potential or current generated by the interaction of the electrolyte with an electrode
• pH-metry or pH measurement.:
Students understand how to measure pH of different solution by using pH-meter.
A pH meter measures the hydrogen ion activity in a solution, indicating its acidity or alkalinity, by measuring the electric potential difference between a measuring electrode (usually a glass electrode) and a reference electrode. This potential difference is then converted into a pH value.
• Colorimetry or Spectrophotometry:
Students explore how to use colorimetry or spectrophotometry to measure the absorbance of coloured electrolyte solutions and relate it to their concentration.
Activity Schedule and Details
The activity was carried out over five days in chemistry laboratory. Students performed experiments on different instrument like Conductometer , Potentiometer , Colorimeter, pH-meter. Students prepared the experimental script to write and record their observations and also plotted the graph of readings. They concluded their results according to their observations. The sessions were structured as follows:
Day 1: Introduction to Electrolytes ,
Introduction to different analytical techniques
Calibration of all the instruments.
Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water. These solutions conduct electricity due to the mobility of the positive and negative ions ,which are called cations and anions respectively.
Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution.
For example, NaCl, HNO3, HClO3, CaCl2 etc. are strong electrolytes. An ionization can be represented by
NaCl(s)→Na+(aq)+Cl−(aq)
Since NaCl is an ionic solid (s), which consists of cations Na+ and anions Cl−, no molecules of NaCl are present in NaCl solid or NaCl solution. The ionization is said to be complete. The solute is one hundred percent (100%) ionized. Some other ionic solids are CaCl2, NH4Cl, KBr, CuSO4, CH3COONa (sodium acetate), CaCO3, and NaHCO3 (baking soda).
Small fractions of weak electrolytes, molecules ionize when dissolve in water. Some neutral molecules are present in their solutions. For example, NH4OH (ammonia), H2CO3(carbonic acid), CH3COOH (acetic acid) and most organic acids and bases are weak electrolytes. The following ionization is not complete,
H2CO3(aq)⇌H+(aq)+HCO−3(aq)
Estimating electrolyte concentrations in mixtures can be done using various methods, including electrical conductivity measurements, spectrophotometry, and electrochemical techniques, each with its own advantages and limitations.
Day 2: To determine the concentration of an electrolyte by Conductometry.
• Electrical Conductivity:
o Principle: Electrolyte solutions conduct electricity, and the conductivity is directly related to the concentration of ions.
o Technique: Measure the electrical conductivity of the mixture using a conductivity meter or similar device.
o Advantages: Relatively simple and fast, can be used for a wide range of electrolytes.
o Limitations: Can be affected by temperature and the presence of other conductive substances.
Day 3: To determine the concentration of an electrolyte by Potentiometry.
Principle: Measure the potential or current generated by the interaction of the electrolyte with an electrode.
Techniques:
Ion-Selective Electrodes (ISEs): Measure the concentration of specific ions in the mixture.
Potentiometry: Measure the potential of an electrode in the mixture.
Amperometry: Measure the current generated by the electrochemical reaction.
• Advantages: Can be highly specific and sensitive, can be used for a wide range of electrolytes.
• Limitations: Can be affected by the presence of other interfering substances, may require complex equipment.
Day 4: To determine the concentration of an electrolyte by using pH-meter.
A pH meter measures the hydrogen ion activity in a solution, indicating its acidity or alkalinity, by measuring the electric potential difference between a measuring electrode (usually a glass electrode) and a reference electrode. This potential difference is then converted into a pH value.
Students used different soil sample from various part of our district to check its pH level like farm soil, urban area soil ,rural area soil, river soil , industrial area soil.
Students also check pH level of some juices , drinking water from various areas.
Day 5: To determine the concentration of an electrolyte by using colorimeter or spectrophotometer.
o Principle: Certain electrolytes absorb light at specific wavelengths, allowing for concentration determination using Beer-Lambert's Law.
o Technique: Measure the absorbance of the mixture at the appropriate wavelength using a spectrophotometer.
o Advantages: Can be highly sensitive and accurate, can be used for a wide range of electrolytes.
o Limitations: Requires the electrolyte to have a characteristic absorption spectrum, may require complex sample preparation.
Participants and Feedback :
The workshop was attended by 130 B.Sc. II year SEM IV students from various groups, including PCM, PHG, MEB, and BIO. The students actively participated in the sessions and showed great enthusiasm in learning new analytical chemistry experiments. The feedback received highlighted the usefulness of the activity in enhancing their analytical and practical skills.
Key Contributors
Dr. G. V. Korpe – Principal, Shri Shivaji Science College, Amravati
Dr. P. R. Mandlik – Head, Department of Chemistry
Dr. D. D. Khedkar – Coordinator, DBT Star Project
Dr. V. B. Khobragade & Ms. K.V. Kumbhalkar – In-Charge of the Activity
Conclusion :
The five-day DBT Star College Activity successfully provided students with valuable skills in analytical chemistry and graphical analysis. By integrating modern technology into physical chemistry education, the program enhanced students’ understanding and application of theoretical concepts. The Department of Chemistry plans to continue such initiatives to further enrich students’ academic and research capabilities.
Outcomes:
- Students learn that electrolytes are substances that conduct electricity when dissolved in a solvent, due to the presence of charged ions.
- Students come to know the different Analytical Techniques for Concentration Determination of mixtures .
- Students understand how conductivity measurements can be used to determine the concentration of electrolyte solutions based on their ability to conduct electricity.
- Students understand how to measure the potential or current generated by the interaction of the electrolyte with an electrode.Students understand how to measure pH of different solution by using pH-meter.
- Students explore how to use colorimetry or spectrophotometry to measure the absorbance of coloured electrolyte solutions and relate it to their concentration.
Photos:
 students performing experiment |  students performing experiment |
 students performing experiment |  students performing experiment |
 students performing experiment |  Group photo |
Attendance Sheet:
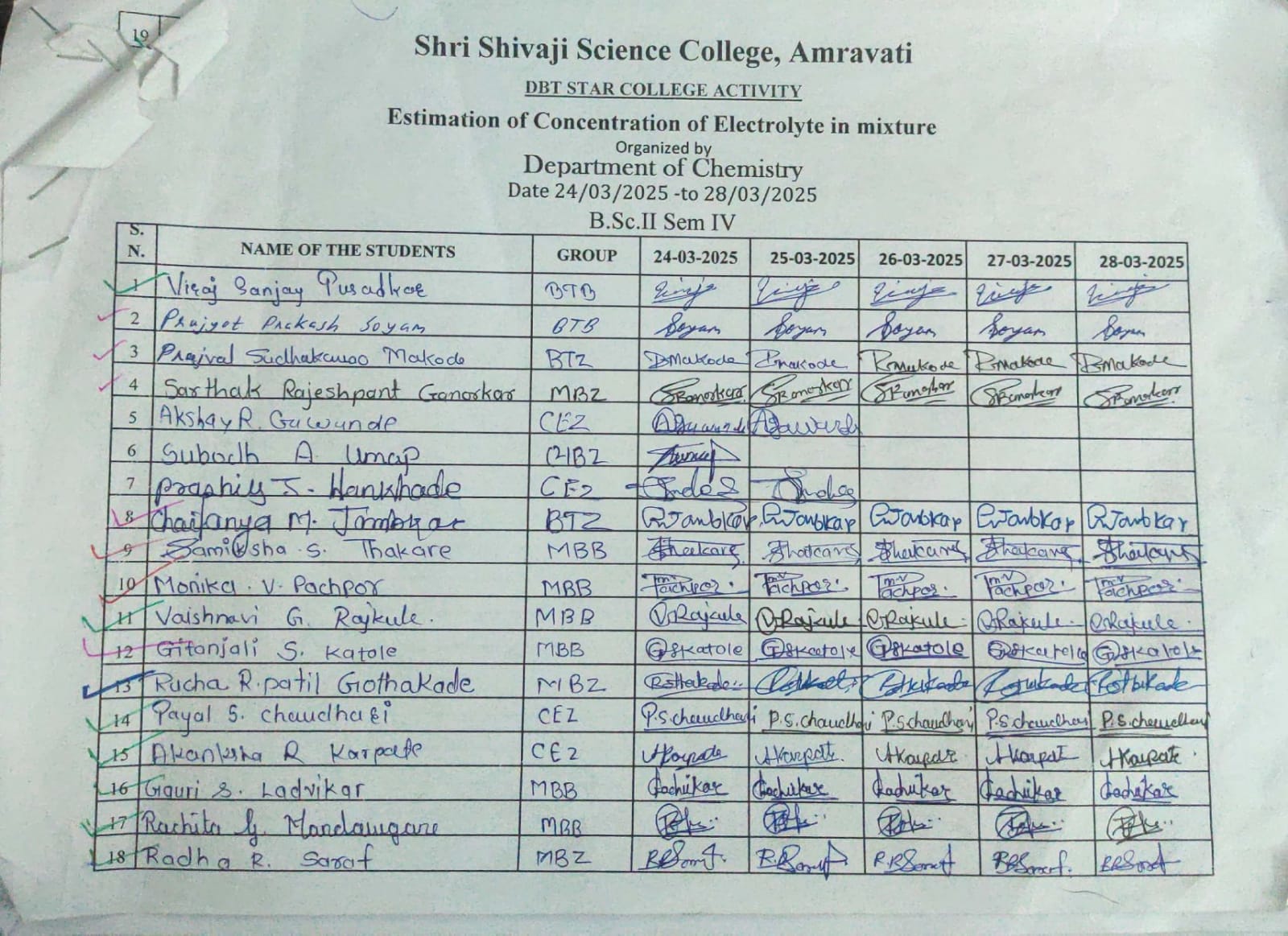 |
 |
 |
 |
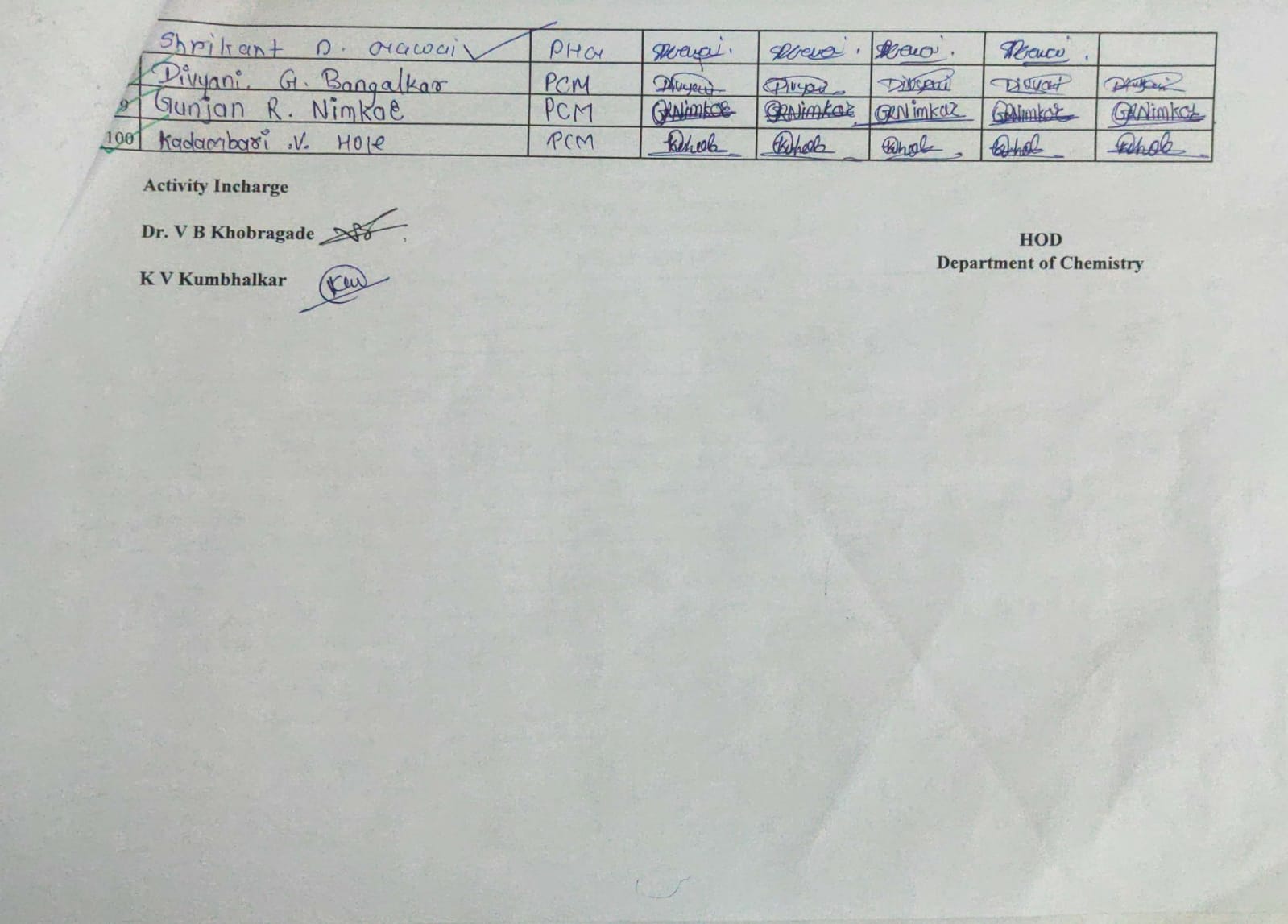 |